 Lithium-ion batteries, which power all kinds of portable electronic devices such as mobile phones, computers, tablets, and have even appeared in cars, motorcycles, bicycles and aerial vehicles such as drones in recent years, are undoubtedly one of the greatest inventions of recent years. Lithium-ion batteries, which have a huge impact on our social life even if we are not aware of it, demonstrated their importance by winning the Nobel Prize in Chemistry for their inventors in 2019. So why are these batteries so important? What did they achieve that previous batteries could not?
Lithium-ion batteries, which power all kinds of portable electronic devices such as mobile phones, computers, tablets, and have even appeared in cars, motorcycles, bicycles and aerial vehicles such as drones in recent years, are undoubtedly one of the greatest inventions of recent years. Lithium-ion batteries, which have a huge impact on our social life even if we are not aware of it, demonstrated their importance by winning the Nobel Prize in Chemistry for their inventors in 2019. So why are these batteries so important? What did they achieve that previous batteries could not?How do batteries work?
First of all, let’s start with the batteries. Batteries are devices that store energy chemically and convert this energy into electrical form. All batteries work on more or less similar principles. Batteries have two main components, one called the anode and the other called the cathode. The anode (negative electrode) is the electrode where the oxidation reaction occurs, that is, the anode material is oxidized by losing electrons. The cathode (positive electrode) is the electrode where the reduction reaction occurs, that is, the materials used in the cathode are reduced by taking electrons. Between the anode and cathode is a substance called electrolyte, which allows the transport of ions, that is, electrically charged particles. The conductive wire connecting the electrodes allows electrons to flow from the anode to the cathode. In this way, the circuit is completed, electric current is generated and the device works.
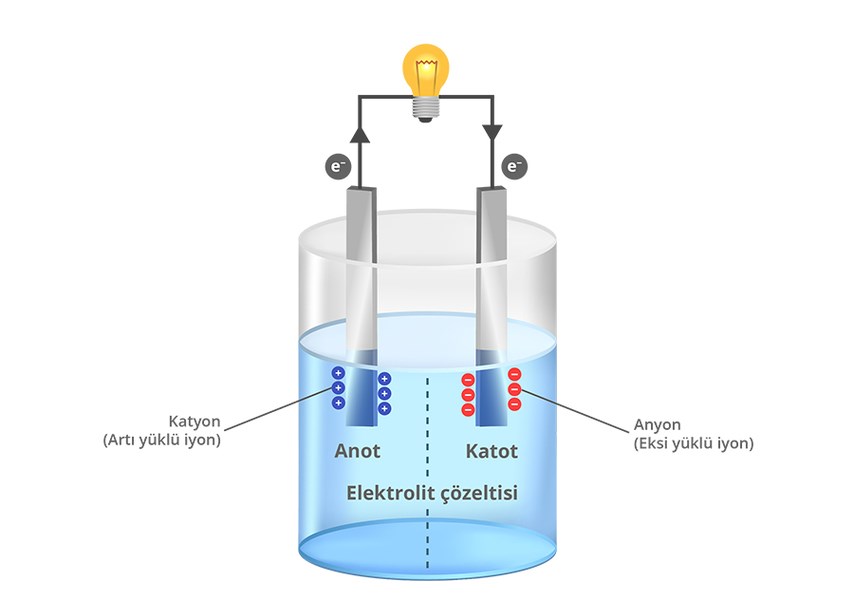 Although knowledge about batteries and electricity goes back further, the first battery was developed by Italian physicist Alessandro Volta. By placing sponges soaked in salt water between copper and zinc plates, Volta succeeded in obtaining electric current. Thus, the first electrochemical battery, called the Voltaic cell, was discovered.
Although knowledge about batteries and electricity goes back further, the first battery was developed by Italian physicist Alessandro Volta. By placing sponges soaked in salt water between copper and zinc plates, Volta succeeded in obtaining electric current. Thus, the first electrochemical battery, called the Voltaic cell, was discovered.lead acid batteries
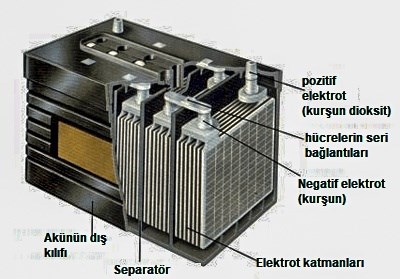 Many types of batteries have been developed since Volta’s invention. However, the most important of these were lead acid batteries. Lead acid batteries, developed by French physicist Gaston Plante in 1859, are the first rechargeable batteries. In the battery, which consists of a lead anode and a lead dioxide cathode immersed in sulfuric acid, electrons in the anode are released when both electrodes react with the acid. At the cathode, the same reaction consumes electrons. Thus, electrons flow from the anode to the cathode, producing electric current. Lead acid batteries work on the same basic principle even today. Even though their energy density and lifespan are low, they still serve us today thanks to their affordable prices.
Many types of batteries have been developed since Volta’s invention. However, the most important of these were lead acid batteries. Lead acid batteries, developed by French physicist Gaston Plante in 1859, are the first rechargeable batteries. In the battery, which consists of a lead anode and a lead dioxide cathode immersed in sulfuric acid, electrons in the anode are released when both electrodes react with the acid. At the cathode, the same reaction consumes electrons. Thus, electrons flow from the anode to the cathode, producing electric current. Lead acid batteries work on the same basic principle even today. Even though their energy density and lifespan are low, they still serve us today thanks to their affordable prices.What makes lithium-ion batteries special?
Many types of rechargeable batteries were developed after lead acid batteries. Batteries such as nickel cadmium and nickel metal hydride have an important place in our lives. However, they all had a major flaw: Their low energy density. This is the most important reason why lithium batteries are so important and why their inventors won the Nobel Prize. It can provide more energy with less volume and weight than other batteries. This energy density comes from the chemical properties of lithium metal. Lithium, located just below hydrogen in the first group of the periodic table, is the metal with the lowest specific gravity. In addition to its lightness, the fact that it is a highly reactive element and can easily donate electrons to form lithium ions makes it a very good battery material.
History of lithium-ion batteries
Many scientists contributed to the development of lithium-ion batteries, which date back to the 1960s. In a twist of history, oil giant ExxonMobil made great contributions to the development of lithium batteries. In 1976, M. Stanley Whittingham, working at ExxonMobil, developed the first rechargeable lithium-ion battery. The battery produced by the researcher, who used titanium sulfur in the cathode and lithium metal in the anode, was both very expensive and had to work in an airless environment. The battery reacted with air and produced hydrogen sulfide, a poisonous gas. Therefore, it could not become widespread.
In 1979, John B. Goodenough of Oxford University and Koichi Mizushima of the University of Tokyo developed the first lithium-ion battery using lithium cobalt dioxide (LiCoO2) as the cathode material. Since lithium cobalt dioxide is a stable cathode material that provides lithium ions, the necessity of using lithium metal on the anode side has been eliminated. Thus, the use of stable and easy-to-use anode materials became possible. This development paved the way for the commercialization of lithium-ion batteries.
In 1985, Akira Yoshino demonstrated that lithium ions could enter the carbonaceous material and developed a rechargeable lithium ion battery using acetylene black at the anode and lithium cobalt oxide at the cathode. The use of carbon in the anode dramatically increased the safety of lithium cobalt oxide and enabled Sony to introduce the first commercial battery 5 years later.
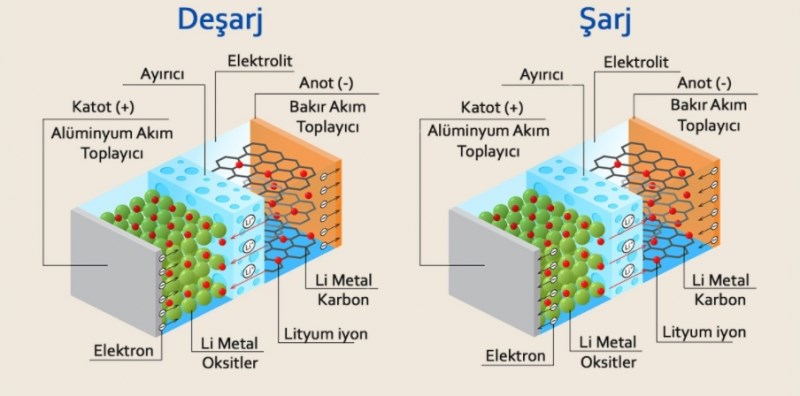
In 1990, Rachid Yazami of the French National Center for Scientific Research collaborated with Sony to develop a graphite anode and liquid electrolyte for lithium-ion batteries and eventually discovered the “magic” ethylene carbonate solvent. With this discovery, the energy density of lithium-ion batteries was doubled (155 Wh/kg) by using soft carbon as the anode material.
In 1991, Sony and Japanese chemical company Asahi Kasei began selling the first commercial rechargeable lithium-ion batteries. The head of the team that developed commercial batteries was Yoshio Nishi.
In 2019, M. Stanley Whittingham, who developed the first lithium-ion battery, John B. Goodenough, who used lithium cobalt oxide as the cathode material for the first time, and Akira Yoshino, who showed that carbon could be used as an anode material, were awarded the Nobel Prize in Chemistry for their contributions to lithium-ion batteries.
Lithium ion battery types
Countless scientists, whom we have omitted, have contributed to the development of lithium-ion batteries and continue to do so. Many types of lithium batteries have been developed as a result of many studies and testing of chemical formulas containing numerous compounds since the 1970s. Now let’s talk about these battery types.
Lithium cobalt oxide (LiCoO2) – LCO
As we mentioned above, lithium cobalt oxide batteries (LCO) are the first commercially developed lithium ion batteries. There is lithium cobalt oxide in the cathode and carbon graphite in the anode. LCO batteries, which have high energy density, have relatively low lifespan and low thermal stability. They also fall short of providing concentrated power. LCO batteries are generally seen in electronic devices such as mobile phones, tablets and laptops. Since LCO batteries contain high amounts of cobalt, they have begun to be replaced by nickel manganese cobalt (NMC) and Nickel cobalt aluminum oxide (NCA) batteries, which contain less cobalt.
Lithium manganese oxide (LiMn2O4) – LMO
LMO batteries, which first came into commercial use in 1996, take their name from the lithium manganese oxide compound used as the cathode material. LMO batteries have low internal resistance and the capacity to cope with high current, as ion flow on the electrode is facilitated thanks to their three-dimensional spinel structure architecture. LMO batteries with high thermal stability are among the safe lithium batteries. However, both charging cycle and calendar life are low compared to other batteries. LMO batteries have a third lower capacity than LCO batteries. Therefore, it is widely used by blending it with NMC batteries in order to increase energy density. This type of battery was used in vehicles such as the Nissan Leaf, Chevy Volt and BMW i3. While the LMO part of the batteries (30%) provides acceleration thanks to its ability to deliver high current, the NMC part provides long range.
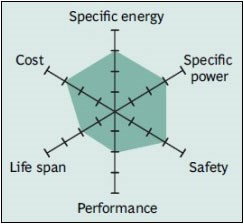
Lithium nickel manganese cobalt oxide (LiNiMnCoO2) – NMC
NMC batteries are one of the most successful lithium-ion battery types used today. Batteries that use a combination of nickel manganese and cobalt in the cathode have high power and energy density. NMC batteries, which have graphite on the anode side like other lithium ion batteries, can be further increased by adding silicon. However, in this case, power capacity and battery life are sacrificed.
Nickel is an important element in increasing the energy density of batteries, but it has low stability. Manganese, on the other hand, has low internal resistance thanks to its spinel structure, but its energy density is low. When these two metals are combined, they increase each other’s strength.
NMC type batteries are the battery type commonly used in electric vehicles. They are named according to the ratio of the metal used. For example, batteries using 5 units of nickel, 3 units of manganese, and 2 units of cobalt are called NMC532. Since cobalt is an expensive and limited element, manufacturers are trying to reduce the amount of cobalt. For this reason, NMC811 type batteries with low cobalt content have become widespread today.
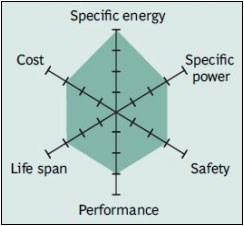
Lithium iron phosphate (LiFePO4) – LFP
LFP batteries were developed in 1996 at the University of Texas as a result of the discovery that phosphate could be used as a cathode material. Lithium phosphate provides low internal resistance and good electrochemical performance in batteries. The main feature of LFP batteries is that they can deliver high current and have a long life. In addition, it is among the thermally stable and safe batteries.
LFP batteries are more resistant to stress at high charge levels than other lithium-ion batteries. Although they are at a lower voltage level than other lithium-ion batteries at 3.2 volts, they can deliver this voltage smoothly. The biggest disadvantage of LFPs is their low energy density. However, due to their other advantages and low production costs, they are increasingly used in automobiles. Due to their long life, they have become the most preferred battery type, especially in stationary storage.
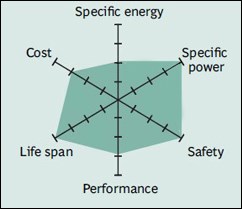
Lithium nickel cobalt aluminum oxide (LiNiCoAlO2) – NCA
NCA batteries, which have been used for special applications since 1999, have high energy density due to their high cobalt level, just like NMC. Batteries with relatively high power and life receive poor marks in terms of safety and cost. Panasonic batteries used in Teslas are NCA type.
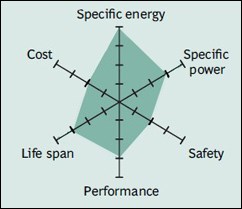
Lithium titanate (Li2TiO3) – LTO
Generally, lithium-ion batteries take their name from the cathode material. However, LTO batteries are an exception, taking their name from the anode material. LTO batteries, known since the 1980s, contain lithium titanate, a titanium compound, in the anode instead of graphite. LMO or NMC is used in the cathode. LTO batteries, which can provide high power and charge quickly, have a very long life. They are also among the safest batteries. They also perform very well in cold weather, but their energy density is the lowest among lithium-ion batteries. Moreover, they are very expensive. Its usage areas are listed as special-purpose electric powertrains, UPSs and solar panel street lights.
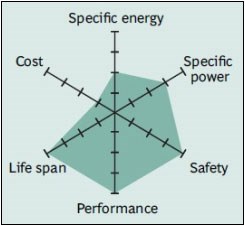
Comparison of batteries
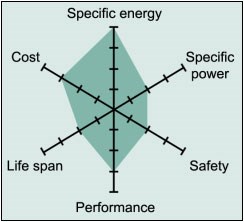
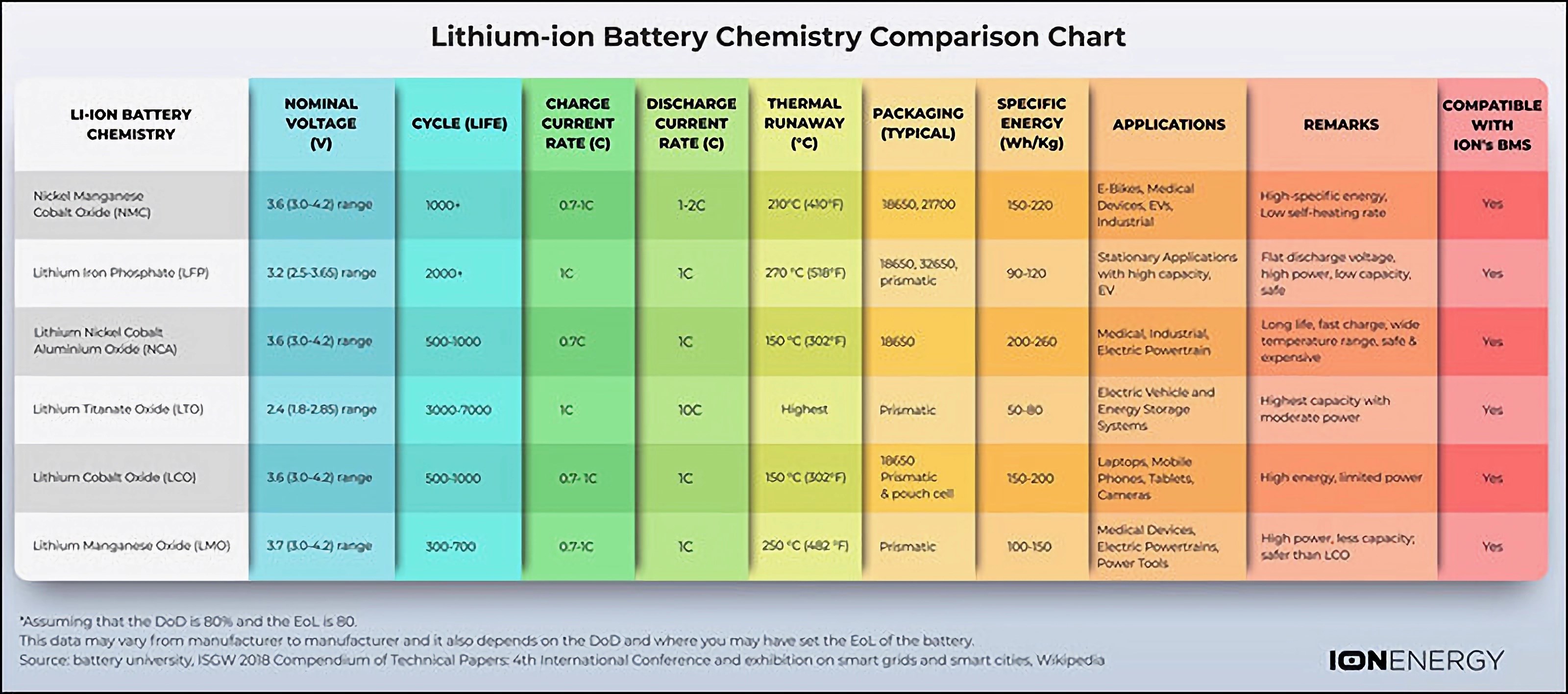
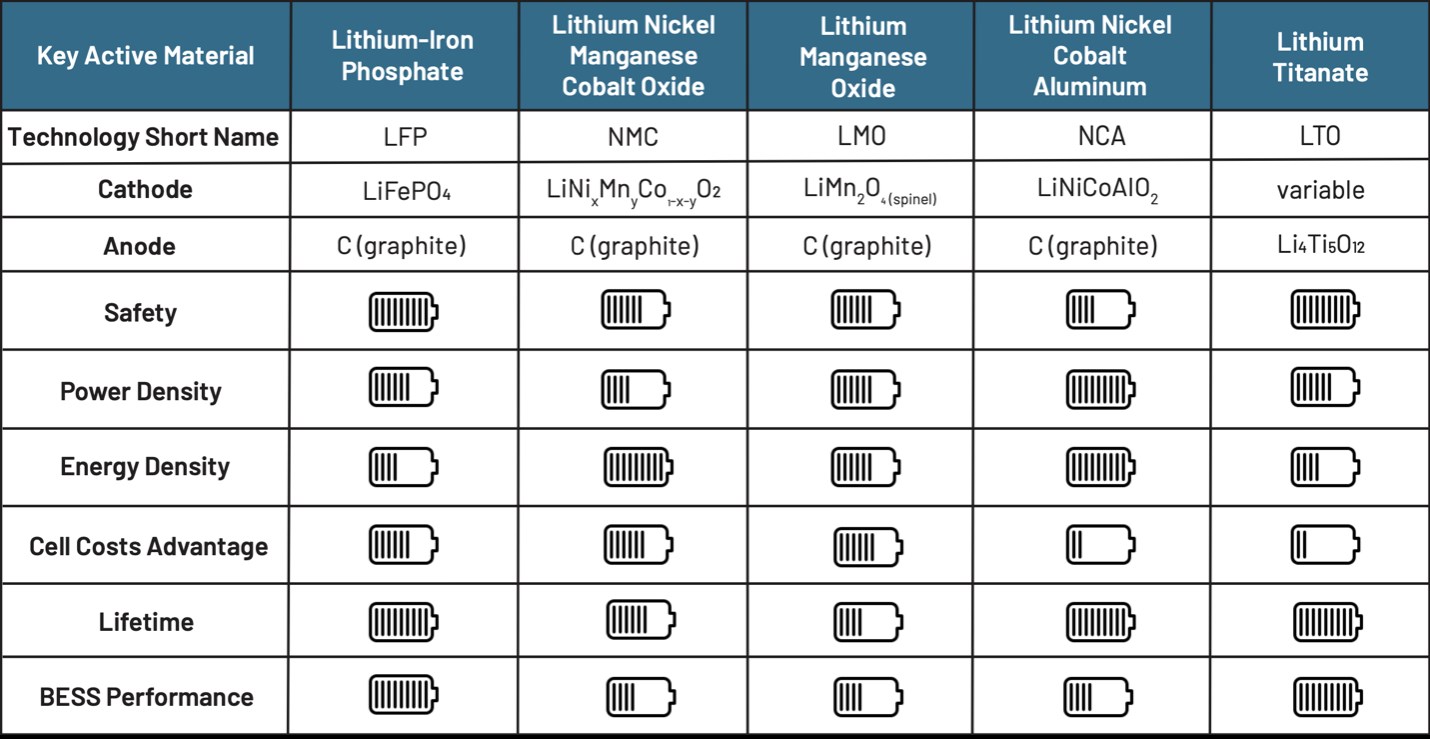 If we compare lithium-ion batteries, NCA batteries are at the top in terms of energy density. However, LMO and LFP batteries stand out in terms of the power they can provide. LTO batteries, on the other hand, stand out with their very long life and cold performance despite their low energy density. NMC batteries stand out from others with their average and above performance in all areas. LFP batteries also differ from others with their cost, long life and safety parameters. In summary, each battery type finds more or less usage areas depending on its advantages and disadvantages.
If we compare lithium-ion batteries, NCA batteries are at the top in terms of energy density. However, LMO and LFP batteries stand out in terms of the power they can provide. LTO batteries, on the other hand, stand out with their very long life and cold performance despite their low energy density. NMC batteries stand out from others with their average and above performance in all areas. LFP batteries also differ from others with their cost, long life and safety parameters. In summary, each battery type finds more or less usage areas depending on its advantages and disadvantages.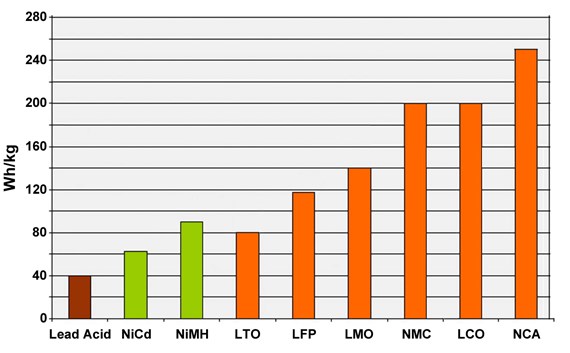
 Today, there is a great need for batteries due to the trend towards renewable energy, electrification and electro mobility. For this reason, studies are being conducted on batteries that are both lithium-based and use other materials, with higher capacity, longer life and cheaper. Studies continue on many promising alternative battery types such as lithium ion batteries with silicon anode, solid state batteries, sodium ion and lithium sulfur batteries. We cannot know which type of batteries will dominate in the future, but it is obvious that lithium-ion batteries have a very important place in our lives today.
Today, there is a great need for batteries due to the trend towards renewable energy, electrification and electro mobility. For this reason, studies are being conducted on batteries that are both lithium-based and use other materials, with higher capacity, longer life and cheaper. Studies continue on many promising alternative battery types such as lithium ion batteries with silicon anode, solid state batteries, sodium ion and lithium sulfur batteries. We cannot know which type of batteries will dominate in the future, but it is obvious that lithium-ion batteries have a very important place in our lives today.